How Does Atomic Explain the Difference Between Spectral Lines
The characteristics of the Atomic Spectrum are observed as. Spectral series are crucial in Astronomical Spectroscopy.

Formation Of Spectral Lines Astronomy
The Atomic Spectrum should be a pure line Spectrum.
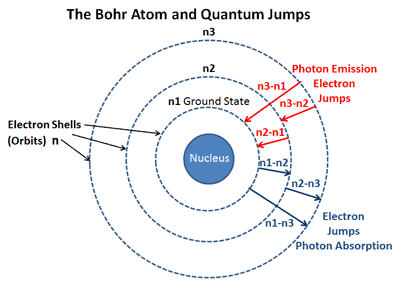
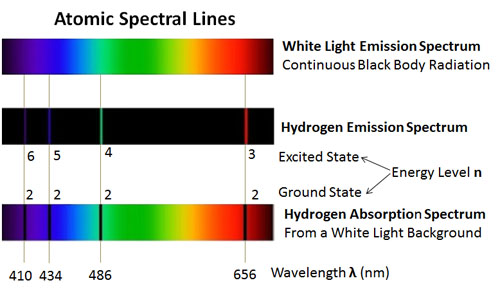
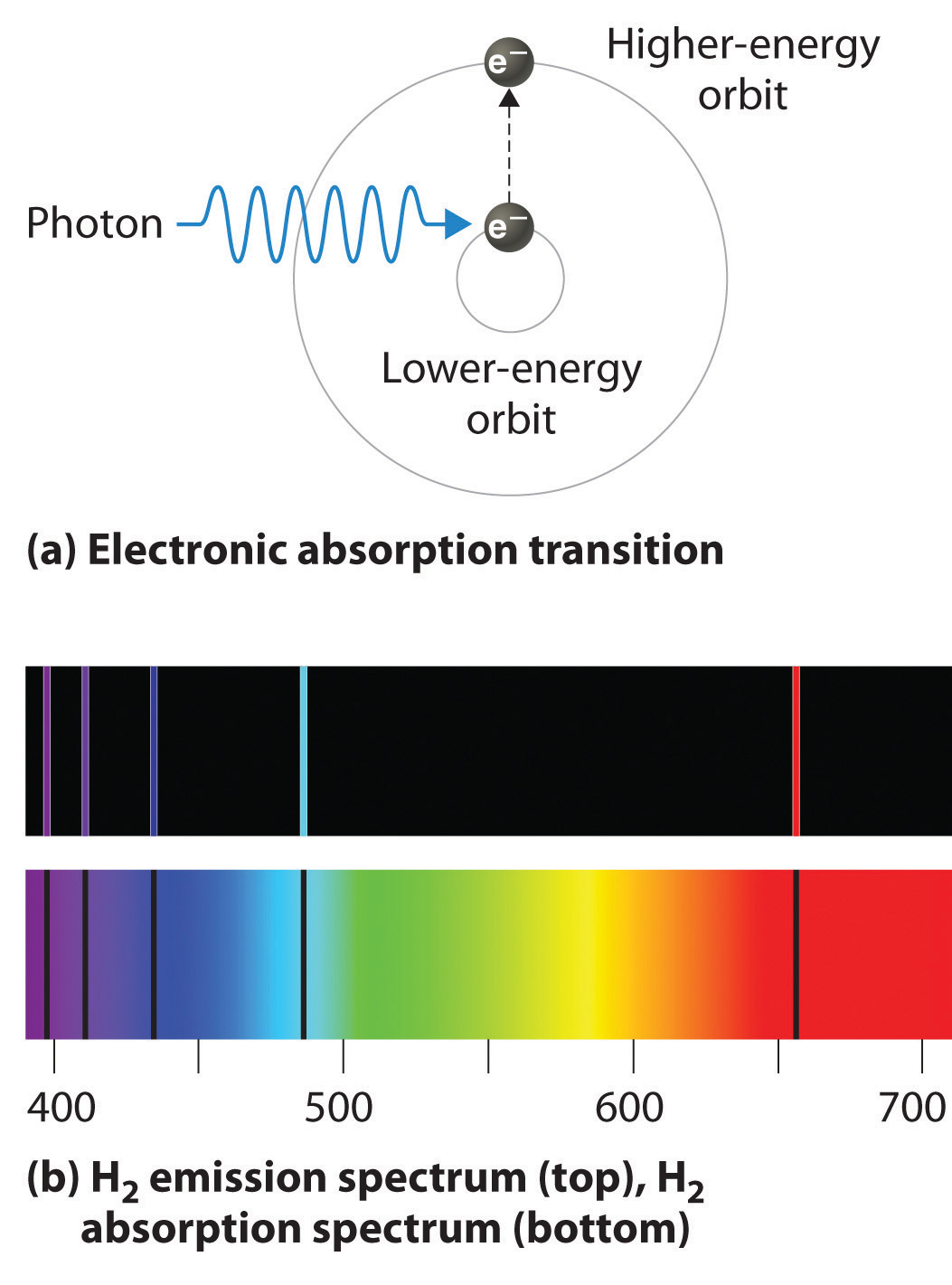
. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum whereas an absorption spectrum has dark-coloured lines in the spectrum. Distinct spectral lines rather than an infinite range of energies and hence white light are a result of only specific transitions being allowed which implies a set of distinct and unvarying energy levels.
Why can line spectra be used to identify individual elements. What is the wavelength of this line. Accordingly what is the consideration of nucleus to explain theory of hydrogen atom.
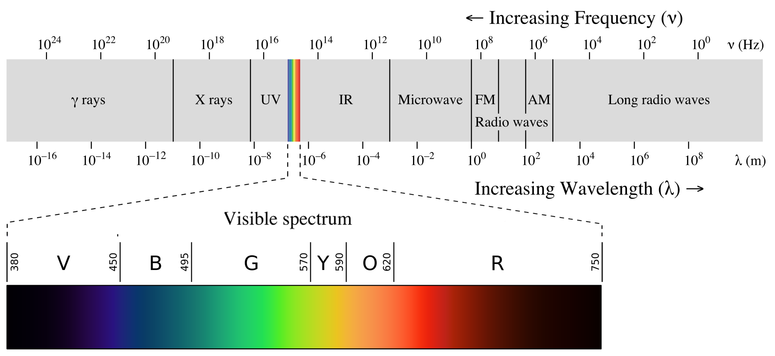
Note that spectral lines can also occur in other regions of the electromagnetic spectrum. Classical models of the atom before quantum mechanics couldnt explain why there should only be certain electron energy levels. It is important to understand the whole context of electromagnetic spectra absorption spectra and emission spectra.
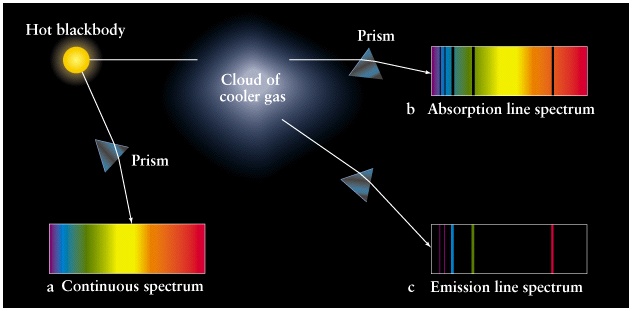
The Bohr modelfails to describe the absorptionemission spectra of atoms that are more complex than hydrogen ie. An absorption spectrum is created when energy is absorbed by atoms of an element and constitutes dark lines in the spectra. Explain the difference between emission and absorpation spectra.
This browser does not support the video element. Secondly a different ques. The visible spectral lines in the hydrogen emission Spectrum are caused by Atomic transitions between distinct Energy levels.
Molecular spectra have three possible types of transitions which are in order of increasing energy 1 rotations in the radio wave range 2 vibrations in the infrared range and 3 electronic in the ultraviolet-visible range. An emission spectrum is created when energy is emitted or released. Why do the emission lines vary in intensity.
It constitutes colored lines in the visible spectra. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. Line spectrum on the other hand only contains a few lines wavelengths.
Atomic Spectra Analysis Questions 1. The emission spectrum of a chemical element or compound is the series of lines that represent the wavelengths of electromagnetic radiation emitted by that chemical. Why do lines in the emission and absorption spectra appear at the same wavelength.
Why do the emission. The main difference between continuous spectrum and line spectrum is that line spectra can be seen as either isolated emission lines or absorption lines with huge gaps between them whereas continuous spectra do not contain gaps and can be produced by superimposing the emission and absorption spectra of the same element. These observed spectral lines are due to the electron making transitions between two energy levels in an atom.
Further theres the so-called fine structure splittings of spectral lines which is due to the spin of electrons which the Bohr modeldid notaccount for. Characteristics of Atomic Spectrum. Atomic spectra show only electron transitions.
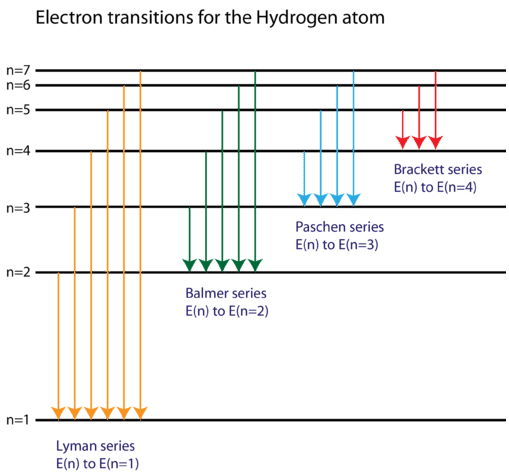
To what series does the spectral lines of atomic hydrogen belong of its wave number is equal to the difference between the wave numbers of the following two lines of the Balmer series 4 8 6. The key difference between hydrogen and helium emission spectra is that the helium emission spectrum plu. Here you should distinguish between two completely different questions which may initially seem similar.
What is the difference between a molecular absorption spectrum and an atomic absorption spectrum. The emission spectrum of atomic hydrogen has been divided into a number of spectral series with wavelengths given by the Rydberg formula. Atoms can absorb some wavelengths when subjected to electromagnetic radiation and present given absorption lines.
A spectral line is like a fingerprint that can be used to identify the atoms elements or molecules present in a star galaxy or cloud of interstellar gasIf we separate the incoming light from a celestial source using a prism we will often see a spectrum of colours crossed with discrete lines. What is the difference between absorption spectra vs. It s also due to electromagnetic radiation.
Spectra has more lines than that of the hydrogen emission spectrum plu. Explain how spectral lines formed. One of these questions which set out here.
The Atomic Spectrum should be the. 1 n m and 4 1 0. By atoms of an element.
An emission spectra is a spectra when atoms release energy and absorption spectrum is when atoms absorb energy. Explain how spectral lines formed. What is the relationship between the atomic number Z and number of spectral lines of an element N 2.
More differences between absorption and emission spectrum are given below in a tabular column. What is the difference between absorption spectra vs. Why do lines in the emission and absorption spectra appear at the same wavelength.
While the absorption spectral lines appear due to electrons traversing from a lower energy level to a higher level emission lines are a result of electrons going from their higher. Why do lines in the emission and absorption spectra appear at the same wavelength. The emission spectrum of atomic hydrogen has been divided into a number of spectral series with wavelengths given by the Rydberg formula.
Give the difference between photovoltaic emission and.
4 2 Understanding Atomic Spectra Chemistry Libretexts

The Origins Of Three Of The Major Sodium Emission Spectral Lines The Download Scientific Diagram
5 7 Spectral Lines Of Atomic Hydrogen Chemistry Libretexts
Lecture 6 Discrete Spectra Of Atoms
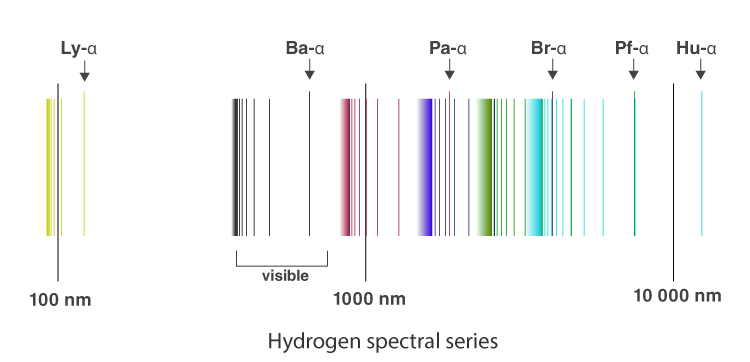
Atomic Spectra Definition Spectral Series Rydberg Formula Atomic Spectroscopy Video And Faqs

Field Spectroscopy Principles Of Structural Chemistry
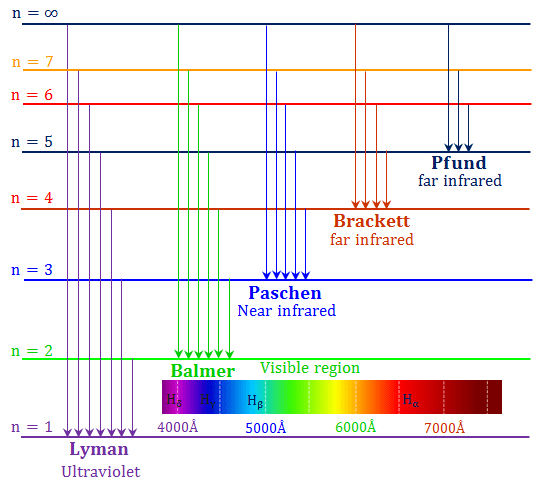
Hydrogen Spectrum Emission Absorption Series Diagram

Optics How Do Astronomers Identify Different Elements From The Combined Emission Spectrum Of Multiple Substances Physics Stack Exchange

6 3 Atomic Line Spectra And Niels Bohr Chemistry Libretexts
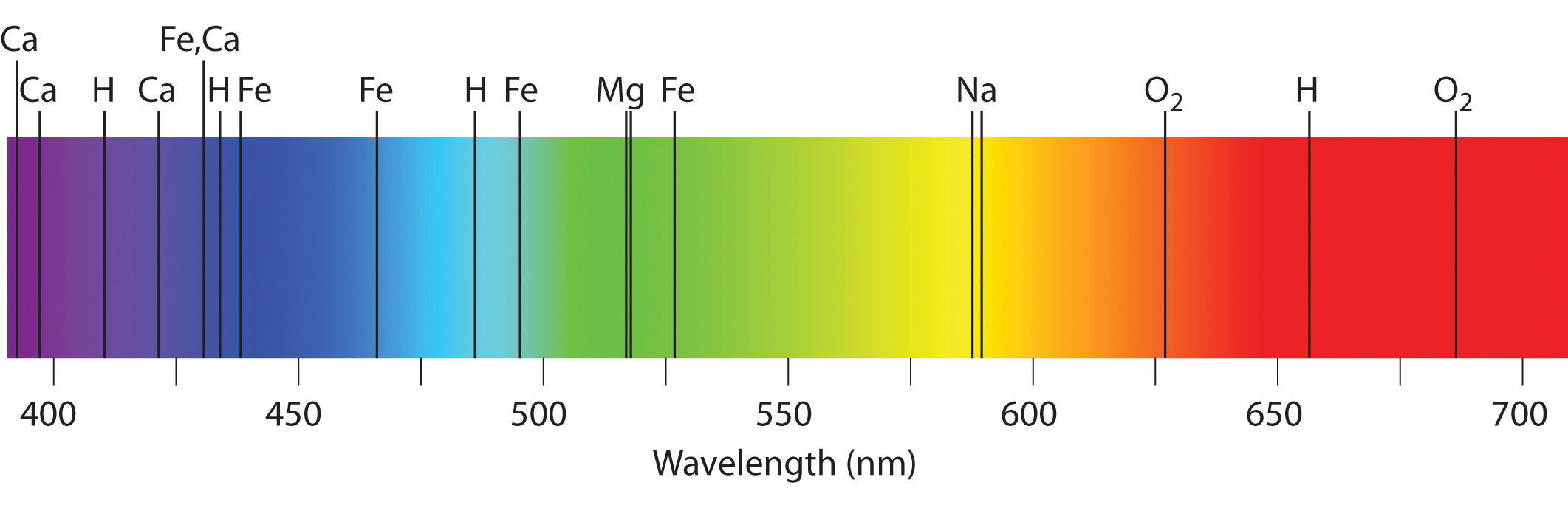
Atomic Spectra And Models Of The Atom

Spectroscopy How Can Every Atom Have Unique Spectral Lines Chemistry Stack Exchange
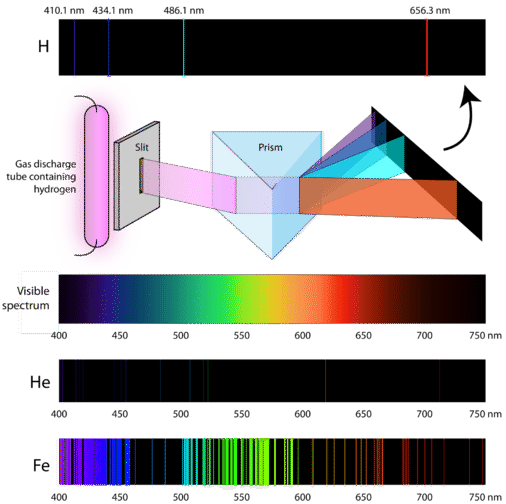
4 2 Understanding Atomic Spectra Chemistry Libretexts

Spectra How To Differentiate Elements That Have Same Spectral Lines In A Star Astronomy Stack Exchange

Comments
Post a Comment